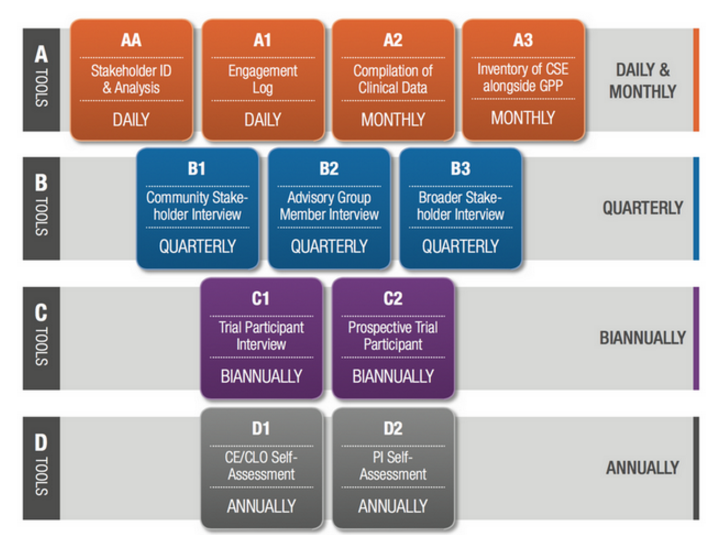
This manual and database presents background and a set of tools to set in motion a culture of monitoring the CSE programs affiliated to HIV and TB related clinical trials. This represents the first CSE M&E toolkit of its kind.
The toolkit, which includes a manual and the database, outlines a set of tools which can assist CSE teams to adopt a culture of systematically monitoring their CSE programs running alongside HIV and TB related clinical trials. This is the first CSE M&E toolkit of its kind.
Visit and browse the Toolkit to find out more
These presentation slides also provide a good insight into the current and future work on the toolkit and on monitoring and evaluating community stakeholder engagement more generally.
The development of this toolkit was led by TB Alliance and AVAC and their consultants Monique Oliff and Kenneth Babigumira, with invaluable contribution from a multi-agency Working Group. This cross-organization effort to develop metrics for CSE aims to contribute to compliment the standards of Good Participatory Research Practices across all forms of biomedical research in low-resource settings. The intention is to enrich the understanding of CSEs potential impact on the outcomes of clinical research.
This work is licensed under a Creative Commons Attribution 4.0 International License


Please Sign in (or Register) to view further.