Mesh theme exploring public and community engagement with vaccine research across four broad themes
Authors: Sian Aggett and the Mesh Editorial Team
INTRODUCTION
The content on this theme page was created following a Mesh workshop exploring public and community engagement with vaccine studies held in autumn 2020. Each of the four workshop sessions had a loose overarching theme chosen to enable participants to unpack some of the complex issues surrounding engaging communities with vaccine research and development. Under each theme below we share projects, thoughts from participants and ways of supporting impactful engagement activities.
1. THE ROLE AND NATURE OF TRUST
We frame ‘trust’ as a relational problem, not a ‘deficit problem’ belonging to public or communities. Who needs to take responsibility for public and community trust in vaccine development and research and how can we coordinate efforts to ensure trust between stakeholders is understood and nurtured?
Using ‘trust’ as a lens, the speakers below reflect on the recent history of vaccine development at a global scale and on how this has both impacted upon and has been influenced through specific case studies.
In the keynote below, Stuart Blume, Emeritus Professor of Science and Technology Studies at University of Amsterdam, gives a brief historical perspective on the role of trust in attitudes to vaccination. He argues there are three distinct roots to current feelings about vaccines and vaccine development:
EXERCISE: Mistrust group exercise
During the workshop, participants took part in an exercise to turn things on their head by asking: "How could we ensure a research project is mistrusted?". Ideas were added across the research cycle from the conception of a study, initiating the research, to the completion of the work (we used Padlet for this). Examples added by participants included not explaining technical terms and using 'jargon' when starting the study, and not responding to community concerns over the results of the research.
Although this may initially seem flippant, the idea is that when we turn each point back the other way, it may give us fresh ideas for ensuring good engagement and strong relationships across a research project.
Heidi Larson, Professor and Director of The Vaccine Confidence Project at London School of Hygiene & Tropical Medicine (LSHTM), follows on from Stuart Blume’s analysis. She addresses the impact of mainstream social media platforms and the rapid spread of rumours about vaccines on levels of public anxiety:
Book: Immunization: How Vaccines Became Controversial by Stuart Blume (2017), Reaktion Books.
Book: Stuck: How Vaccine Rumours Start – and why they don’t go away by Heidi J Larson (2020), Oxford University Press USA.
EXERCISE: Sharing in ‘Peer Learning’ style
When grappling with issues of trust within a vaccine research project, it can be useful to reflect on some of the important relationships and unpick the key issues with a small group of colleagues. This kind of peer learning (see Intrac’s guide to Action Learning Sets) was used in the workshop to look at the role of trust in a series of case studies presented by participants. The idea is that you can draw on the knowledge and skills of a small group of peers to reflect back on actions taken, drawing out learning from those reflections and applying that to future planned activities.
In the short film below Alun Davies, Social Scientist at KEMRI-Wellcome Trust Research Programme in Kenya, gives a session summary and reflections on the theme of trust. Alun notes that wealth disparities between researchers and communities can fuel mistrust and that it is always important to recognise that trust is constantly shifting within populations and can decay easily over time. Alun highlights the importance of early engagement and the need for transparency but critically asks; do engagement and transparency always lead to increased trust?
Mesh Podcast: How can Engagement Practitioners make themselves more Trustworthy in the case of Vaccination? - This Podcast explores the power of trust between communities who are hesitant towards vaccines, and the public health community who are delivering immunisation. Dr Heidi Larson gives an in-depth analysis of the pitfalls and challenges in tackling this issue. Listen to it HERE
Workshop Report: Trust Me I Am a Scientist - This report is based on conversations that took place at the Wellcome Trust’s fifth international engagement workshop in November 2013. It also includes a literature review which focuses on trust in the context of health research in developing countries and highlights a range of challenges affecting levels of trust. Read it HERE
2. THE VALUE AND CHALLENGE OF COLLABORATION
There is an increasing complexity of actors working in vaccine research. The N.G.O/humanitarian sector, the private sector and the public sector might all be collaborating on the same trial. Each can have their own understanding of what engagement ought to be, what conversation frames are important and who ought to be involved. In emergency contexts, multiple trials might be taking place concurrently within the same region. What challenges does this create for engaging local populations?
The resources in this section look at aligning engagement across partnerships and similar geographic areas, and at the synergies and distinctions between different roles such as communications, engagement and social research within the same trial site.
In this presentation Gabrielle Breugelmans, Head of Epidemiology at The Coalition for Epidemic Preparedness Innovations (CEPI), talks about collaboration at an international institutional level. She shares an engagement experience from their Lassa Fever Epidemiology Programme including how collaboration worked within the governance and execution of the project:
CEPINET is a network of institutes, clinical investigators, and social scientists who are committed to the development of effective, culturally-appropriate community engagement models to support vaccine development of CEPI’s priority diseases.
EXERCISE: Aims of engagement
Participants at the workshop were asked “The purpose of engagement with vaccine research is...?”. Identifying over-arching aims at the outset can help ensure alignment between different collaborators. Below is a phrase cloud of the answers submitted by the participants at the Mesh workshop on Mentimeter:

Reflecting on collaboration and Ebola vaccine trials
In the panel session below, chaired by Mark Marchant, Research Fellow at London School of Hygiene and Tropical Medicine (LSHTM), those who were involved in Ebola vaccine trials in Sierra Leone following the 2014 to 2016 outbreak in West Africa, share their experiences and reflect on the challenges and opportunities of collaboration across institutions and implementation partners when it comes to testing a vaccine:
Speakers: Luisa Enria, Assistant Professor at London School of Hygiene and Tropical Medicine (LSHTM); John Johnson, Référent Médical Vaccination et Réponse aux Epidémies at Médecins Sans Frontières (MSF); Robert Kanwagi, Programme Coordinator at Ebola Vaccine Deployment Acceptance & Compliance Program/WV Ireland; and Shelley Lees, Associate Professor at London School of Hygiene and Tropical Medicine (LSHTM)
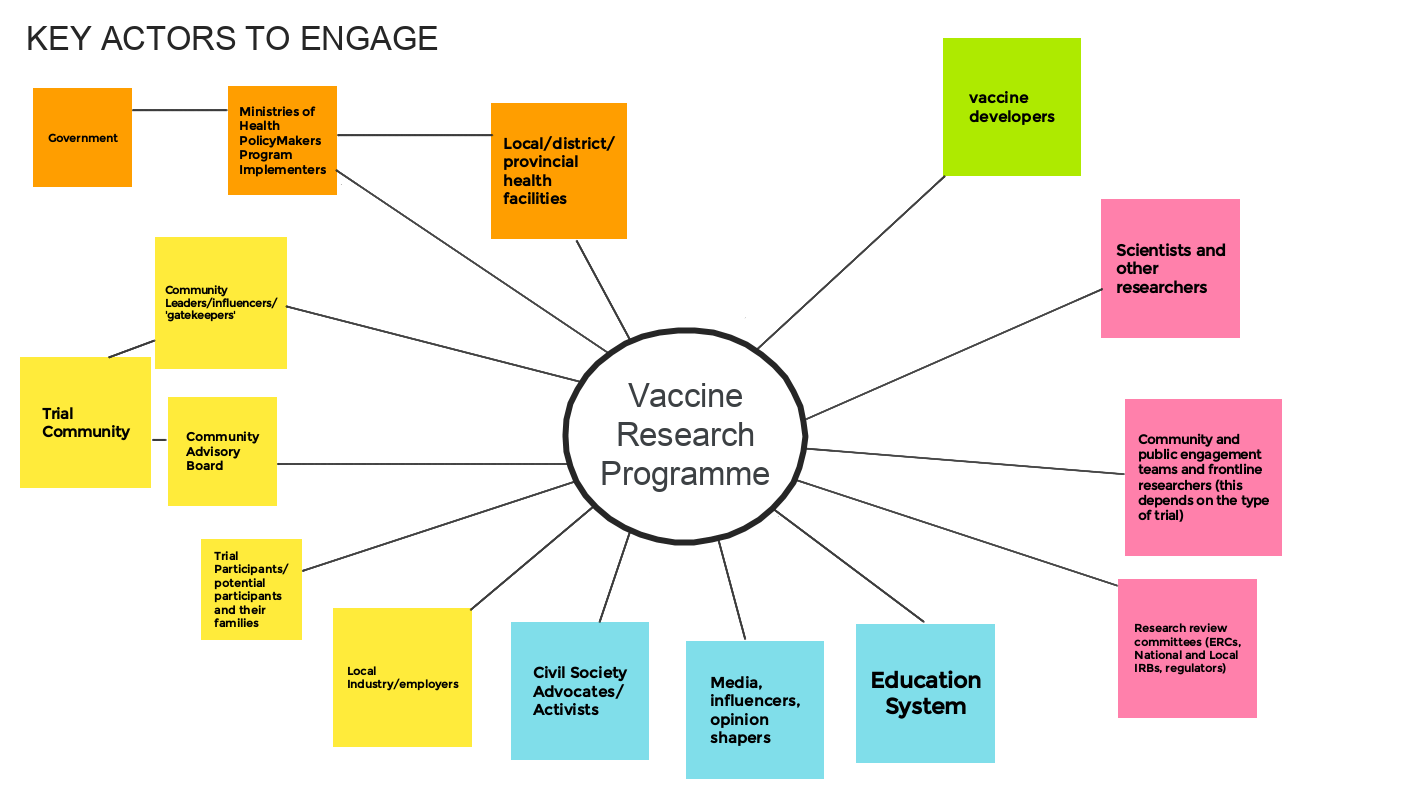
EXERCISE: Identify key 'actors'
Participants within the workshop worked in small groups to explore and identify the key stakeholders or ‘actors’ for engagement work within a vaccine research programme. They were also asked to note why each individual or group need to be engaged. They used a Jamboard to record their thoughts. An example is given below.
In practice this mapping exercise can help in the design of engagement activities and approaches. Once the key actors are identified, along with reasons for engaging them, then channels for listening and responding to each group can be determined.
In the video below Jessica Salzwedel, Program Manager - Research Engagement at AVAC, talks about the power of collaboration and explains the partnerships that AVAC works through on their stakeholder engagement work. She describes the examples of national collaborations and how AVAC’s Good Participatory Guidelines have been adapted for specific contexts:
In this final short film Nancy Lee, Programme Director at Wilton Park, gives a session summary and reflections on the theme of collaboration in research studies. She talks about why collaboration is so important for community engagement and particularly in vaccine development. Nancy also brings out some top tips for successful collaboration gathered during the session:
3. LISTENING TO PUBLIC AND COMMUNITIES
Engagement is often purported to be the way of ensuring that a study is context-sensitive and appropriately targeted. What are the sensory structures and feedback loops that are required to ensure that this is the case? At what stage in the research cycle does engagement need to take place? Through what channels and when might we expect to hear community and public voice?
During the third session, participants explored ways in which trials might create mechanisms to listen and respond to public and communities themselves. Presenters described how community voices are expressed and in what spaces (Community Advisory Boards, advocacy groups, public protest etc), ways to ensure that community voices might inform research processes themselves and whose responsibility it might be to continue relationships and conversations with communities in the long term.
In the keynote below Priya Bahri, Principal Scientific Officer at European Medicines Agency (EMA), looks at ways of listening to stakeholders and the public from a regulatory perspective. She outlines some of the regulators’ mechanisms for listening and describes a media monitoring project looking at HPV vaccines. This film also includes some reflections and questions from Gill Black, Managing Director at Sustainable Livelihoods Foundation, regarding inclusion of those living in marginalised communities and techniques for public dissemination:
Published Literature: ‘Engagement’ of patients and healthcare professionals in regulatory pharmacovigilance: establishing a conceptual and methodological framework - Brown, P., Bahri, P. Eur J Clin Pharmacol 75, 1181–1192 (2019). Access it HERE.
In the case study presentation below Bvudzai Magadzire, Senior Technical Advisor at Village Reach, shares some reflections from their current qualitative participatory study in Mozambique, Bate Papo Vacina! (Let’s get vaccinated!). The project is seeking to understand the barriers to vaccination in the Zambézia province, and uses Photovoice, live messaging, semi-structured interviews and a human-centred design workshop.
Mesh Project Report: Bate-Papo Vacina! (Let's get vaccinated!) - Read this blog style article from the team at Village Reach and look out for new updates on the project HERE.
EXERCISE: Value of listening
Communication of key messages about research work to all major stakeholder groups is obviously very important to the success of a study, but sometimes it can be harder to make a case for using resources for in-depth listening work. Exploring community views and responding to ideas and concerns can be challenging to manage on top of existing planned activities. Below is a quick and light-hearted exercise to show the direct benefit of listening:
- Ask your participants to write the names of three people whom they consider to be good listeners. Check with each person to see if they have written three names (some may find it difficult)
- Ask if anyone has written the name of someone they don't like (usually nobody has!)
- Then ask if the three people they have written fit in any one of these categories: liked by them, loved by them or respected by them. Of course, the response is usually 'yes'. It is likely that even if someone has written the name of someone they do not like, that person will be in the category of being respected.
For those involved in a research study who are perhaps more focussed on building support in local communities, this very quickly makes the point that there is real value in showing a genuine desire to listen to others.
In this next case study presentation Patrick Faley, Social Mobilization Coordinator at Partnership for Research on Ebola Vaccines in Liberia (PREVAIL), outlines the importance of listening to community voices during a vaccine trial, discusses some of the cultural challenges and give recommendations and lessons learned:
The role of Rumour
The video below is an informal discussion between Robert Kanwagi, Programme Coordinator at Ebola Vaccine Deployment Acceptance & Compliance Program/WV Ireland and Noni Mumba, Head of Community Engagement at KEMRI-Wellcome Trust Research Programme in Kenya. The speakers each have experience of the impact of rumours spreading within the community on clinical research. They give some fascinating insights into how and why rumours come about and what can be done to help research to continue:
During this final short film Julio Canario, CEO at Etikos / Instituto de Salud Mental y Telepsicología (ISAMT), gives a summary of the session and the themes of listening and responding. Julio highlights the need for listening within the current international context and references the multiple information sources competing for public attention; often termed as an ‘infodemic’. He highlights the value of Community-Based Participatory Research as a way of carrying out research that has listening incorporated into the approach:
4. SUPPORTING STRONG ENGAGEMENT PRACTICE
The series of videos within this section come from various different global organisations who have worked to develop guidelines, frameworks and toolkits which aim to support strong community and stakeholder engagement either within clinical research or for development practice and humanitarian action. These principle-based resources, and associated implementation tools, are often used by research teams in the design and conduct of new studies.
Discussions in this workshop session reflected on the resources that already exist in this space and explored how we can continue to encourage Good Participatory Practice (GPP) in clinical trials. What works and why? What might be needed and what lessons and opportunities might we capitalise on in developing future guidance? Our reflection on the day also included a call to action for collaboration on developing future support.
In this first film Nick Medhurst, Policy and Advocacy Manager at Good Clinical Trials Collaborative (GCTC), describes this new initiative which is a partnership between Wellcome, the Gates Foundation and the African Academy of Sciences. GCTC is focussed on supporting the development and use of new guidance that promotes and enables rational, proportionate and critical application of Good Clinical Practice principles for clinical trials.
In the keynote presentation below Beatriz Da Costa Thome from Universidade Federal de São Paulo (UNIFESP) outlines some of the learning arising from the creation of the Nuffield Council on Bioethics report on research in global health emergencies. She also shares some experiences from working with communities in Brazil when setting up a serological survey with vulnerable groups:
Report: Research in global health emergencies, Nuffield Council on Bioethics - This report from January 2020 contains the findings of a two year in-depth inquiry into the ethical issues relating to research in global health emergencies. The aim of the report is to identify ways in which research can be undertaken ethically during emergencies, in order to promote the contribution that ethically-conducted research can make to improving current and future emergency preparedness and response. Read it HERE
eLearning Course: Research in global health emergencies: Ethical issues - This specialist short course explores some of the core ethical issues that arise in the conduct of research in global health emergencies – from outbreaks of infectious diseases to natural and human-made disasters. It is based on the report above. Take the course for free HERE.
PART A: Supporting Participatory Practice in Clinical Trials: Adapting GPP Guidelines to contexts
The video below brings together three presentations from key organisations involved in the creation, adaptation and use of GPP Guidelines for health research across HIV prevention, tuberculosis treatment and emerging infectious diseases.
Stacey Hannah, Director of Research Engagement at AVAC, describes the history and current perspective on the Good Participatory Practice Guidelines that were developed by AVAC and UNAIDS. These guidelines provide trial funders, sponsors and implementers with systematic guidance on how to effectively engage with all stakeholders in the design and conduct of trials.
Stephanie Seidel from the TB Alliance describes the adaption of GPP to tuberculous treatment research and outlines a series of challenges and barriers to implementing community engagement within clinical trials including how it is integrated into clinical operations and planning.
Lisa Schwarz, Professor at McMaster University and lead for WHO Task Force on Good Participatory Practice in Emerging Pathogen Research, gives an outline of the Task Force and their development of tips and guides. She also highlights the importance of community engagement within COVID-19 research despite restrictive measures, poor access and time pressures.
Guide: AVAC Good Participatory Practice (GPP) Guidelines - Developed by AVAC and UNAIDS, GPP are intended to provide trial funders, sponsors and implementers with systematic guidance on how to effectively engage with all stakeholders in the design and conduct of biomedical HIV prevention trials. GPP has been adapted to a variety of other research areas and settings. Find out more HERE.
Guide: Good Participatory Practice Guidelines for TB Drug Trials (GPP-TB) - Providing guiding principles and practice standards for stakeholder engagement as an integral part of TB drug trials. Stakeholder engagement refers to any form of consultation, collaboration, and partnership put in place to enable a dialogue between all parties having a stake in a specific trial. Find out more HERE.
Guide: Good participatory practice guidelines for trials of emerging (and re-emerging) pathogens that are likely to cause severe outbreaks in the near future and for which few or no medical countermeasures exist (GPP-EP) - Aimed at those involved in designing, financing, and implementing prevention and treatment trials of emerging or re-emerging pathogens. These are pathogens that are causing or are likely to cause severe outbreaks in the near future. Download HERE.
Toolbox: WHO R&D Good Participatory Practice for COVID-19 clinical Trials - This toolbox was developed for the World Health Organisation COVID-19 Research Roadmap as a joint initiative between the social science and ethics working groups. It provides a “how to” guide, overview, tips and resources. Find out more HERE.
Toolbox: Working with Community Advisory Boards for COVID-19 related clinical studies - Here the WHO Task Force on Good Participatory Practices in Emerging Pathogens (GPP-EP) shares information on establishing and working with different types of advisory groups in the context of COVID-19 clinical studies. Find out more HERE.
PART B: Supporting Engagement in Clinical Trials: Other Approaches
In this next video, we hear from four presenters who discuss supporting engagement through different entry points into the research system including at a structural level, through ethics review and directly with communities.
Jim Lavery, Chair in Global Health Ethics and Professor at Center for Ethics at Emory University, discusses some of his team’s current work including a Gates Foundation-funded project focussing on the role of fair partnerships as a determinant of effectiveness of global health campaigns. He describes how Cohred’s Research Fairness Initiative can be used as framework to inform how organisations can go about building fairness into partnerships.
Cathy Slack, Project Director at HIV AIDS Vaccines Ethics Group (HAVEG) at University of KwaZulu-Natal (UKZN), references the abundance of engagement guidelines and the need for identifying commonalities. Her presentation describes what current international ethics guidelines see as core feature and practices of strong engagement.
Sharon Abramowitz, Consultant to UNICEF Communication for Development, discusses the development of the inter-agency Minimum Quality Standards and Indicators for Community Engagement which is broadly applicable to health studies but not focussed on clinical research. Sharon describes how community engagement can be seen at the centre of various fields of activity in the global context but can have a series of core standards.
Ntando Yola, Community Engagement Lead at Desmond Tutu Health Foundation, shares experiences of operationalising GPP Guidelines in the South African context. He describes their mechanisms for engagement of civil society in biomedical HIV and TB research processes and their learning from the process.
Resources: The Human Engagement Learning Platform (HELP) for Global Health - HELP aims to support global health organisations and programs to translate their ethical aspirations into policy and practice through research and scholarship, training and education, and strategic consulting in five key programs. Find out more HERE.
Published Literature: The roles of stakeholder experience and organizational learning in declining mass drug administration coverage for lymphatic filariasis in Port-au-Prince, Haiti: A case study. Wodnik BK, Louis DH, Joseph M, et al. PLoS Negl Trop Dis. 2020;14(5) (2020). Access it HERE.
Published Literature: Evidence-based health policy: context and utilisation. Dobrow MJ, Goel V, Upshur RE. Soc Sci Med. 58(1):207-17 (2004). Access it HERE.
Published Literature: Strengthening stakeholder engagement through ethics review in biomedical HIV prevention trials: opportunities and complexities. Slack C, Wilkinson A, Salzwedel J, Ndebele P. J Int AIDS Soc. 21 Suppl 7 (2018). Access it HERE.
eLearning Course: AVAC's Strengthening Stakeholder Engagement through Ethics Review – HIV Prevention Trials - Rooted in ethics guidelines, this course provides a condensed overview of core features and practices of ‘engaged’ clinical trials, and how these can be supported in ethics review. It is focused on HIV but relevant to other infectious diseases. Find out more HERE.
Guide: Minimum quality standards and indicators in community engagement – Developed through an inter-agency consultation process supported by UNICEF's C4D, this document sets core minimum standards developed in line with the principles of human rights- and community-based approaches, such as participation, inclusion and accountability. Download the PDF HERE.
In the final video in this theme area Cathy Slack, Project Director at HIV AIDS Vaccines Ethics Group (HAVEG) at University of KwaZulu-Natal (UKZN), gives her reflections and summary of the key themes coming from discussions about ways of supporting strong practice. Cathy concludes that although there is currently a great wealth of guidance on why we should engage and who we should engage with, there is still a question for her about how much we should engage and what is reasonable and justified:
SUMMARY REPORT: Supporting Strong Engagement Practice in Clinical Trials - This report from Hilo Consultants Ltd gives more detail on the key themes arising from this final workshop session. It summarises the advice, suggestions and number of recommendations given by workshop participants for supporting engagement within clinical trials. Download the report HERE
Search the existing articles on Mesh related to vaccines and vaccine hesitancy HERE
FURTHER RESOURCES
Toolkit: Animated Resources Explaining COVID-19 Vaccine Trials - These illustrated slides, created by Alun Davies at KEMRI-Wellcome Trust Research Programme (KWTRP) in Kenya, give a simplified scientific explanation of vaccine development, including the sequence and timeline for clinical studies, ethical review and randomisation. The slides are designed to be easily modifiable, so local scripts and narration can be added to make them relevant to different contexts. Download them HERE
Toolkit: How Vaccines Work - Lifeology “Flashcard” illustrated primer (Open access and also available in Spanish here) – Lifeology is a platform and a community space that brings together scientists, health experts, artists, writers and broader audiences in the creation of mobile-friendly Lifeology mini-courses. See their other COVID-related “flashcard” courses HERE
Animation: COVID-19 | How a vaccine can protect you - A new Eh!woza animation in collaboration with artist, Mitchell Gilbert Messina, and postgraduates from the MRC/NHLS/UCT Molecular Mycobacteriology Research Unit (MMRU). The first in a series about vaccines, this animation describes how the immune system works and vaccine-mediated protection from disease develops. View it HERE
Video: Vaccines and the Immune System - Animated video from University of Glasgow, UK giving an introduction to how the immune system works, the importance of immune memory and how vaccines protect against infectious diseases. For more resources on engaging the public with vaccine immunology visit the British Society for Immunology HERE
Published Literature: Science, theory, and practice of engaged research: Good Participatory Practice and beyond – Journal of the International AIDS Society (Volume 21, Supplement 7, October 2018). Includes 9 papers on the topic.
Published Literature: Application of real-time global media monitoring and ‘derived questions’ for enhancing communication by regulatory bodies: the case of human papillomavirus vaccines – paper from BMC Medicine by Priya Bahri et al - the European Medicines Agency (2017)
Report: Systematic scoping review on social media monitoring methods and interventions relating to vaccine hesitancy – A systematic scoping review by ECDC and the Vaccine Confidence Project summarises knowledge and research on social media and vaccination. (March, 2020)
Published Literature: Strategies for addressing vaccine hesitancy - A systematic review - authored by the SAGE Working Group. This study identifies, describes and assesses the potential effectiveness of strategies to respond to issues of vaccine hesitancy that have been implemented and evaluated across diverse global contexts (October, 2014)
Published Literature: Controversial Ebola vaccine trials in Ghana: a thematic analysis of critiques and rebuttals in digital news – paper from BMC Public Health by Heidi Larson et al. (2017)
Video about controversial Ebola vaccine trials in Ghana - The Vaccine Confidence Project (https://www.vaccineconfidence.org/) (Febuary, 2018)
Toolkit: Engagement for Impact: Monitoring & Evaluation - This user friendly set of quantitative and qualitative monitoring and evaluation tools will allow you to capture, collate and analyze Community and Stakeholder Engagement (CSE) data at the clinical trial site-level
Article: Praise for new public involvement COVID-19 matching service - NHS Health Research Authority (June 2020)– news piece on the matching service set up to connect COVID-19 research teams with public involvement expertise
Toolkit: GPP Training & Implementation Tools - The GPP implementation tools are meant primarily for groups and individuals who are responsible for ensuring that GPP is followed at various levels. The training tools can be used by anyone who wishes to conduct a training or to provide an overview of the GPP guidelines to a secondary audience
eLearning: GPP Online Training Course - a four-month, hands-on eLearning experience that brings the GPP Guidelines to life through interactive online content, case studies, work assignments and online discussions
Toolkit: Stakeholder Engagement Toolkit - developed by FHI360, this is a guide to engaging a wide range of key stakeholders throughout the trial lifecycle
Please add more reports, papers, links, guides/tools by emailing: mesh@tghn.org
This work is licensed under a Creative Commons Attribution 4.0 International License